The new Bluetooth® CO monitor from Bedfont® Scientific Ltd. has helped Stop Smoking Clinics to continue giving essential advice to its patients through remote CO monitoring
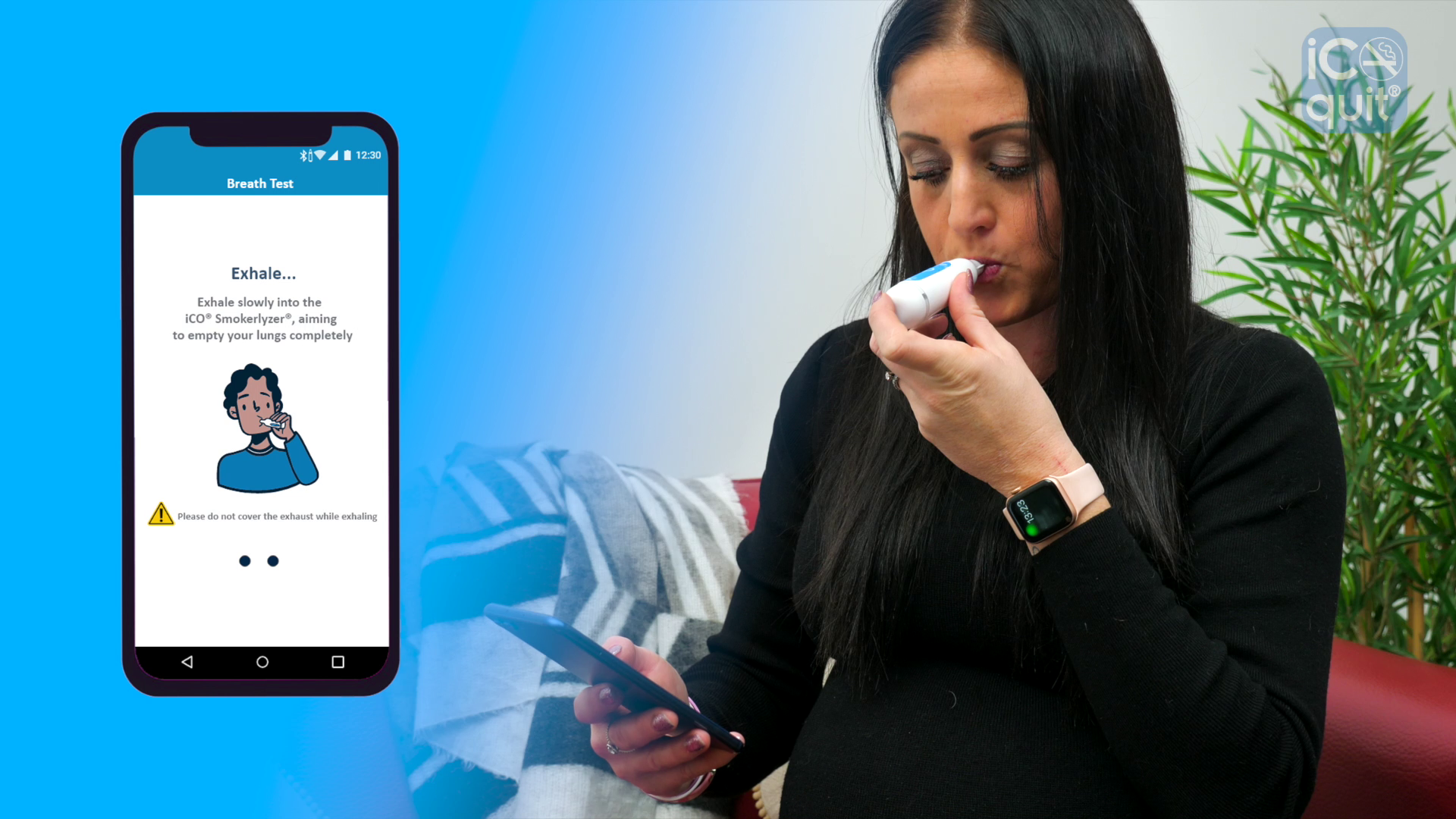
Medical device manufacturer, Bedfont®, has launched its new iCOquit® – a portable, personal Bluetooth® Carbon Monoxide (CO) monitor to help people quit smoking, which is helping Stop Smoking Services to support their patients remotely during the Coronavirus pandemic.

According to the NHS, “Smoking is one of the biggest causes of death and illness in the UK”, responsible for around 78,000 deaths each year, in addition to even more suffering from “debilitating smoking-related illnesses”1. Carbon monoxide monitoring is a very effective tool in smoking cessation; it can validate a person’s smoking status and acts as a great motivational tool for the patient, showing them visible proof of harm caused by tobacco smoking, plus, studies show that smokers who use CO monitoring during their quit attempt are more likely to be successful2.
Despite face-to-face smoking cessation consultations being postponed due to the pandemic, now thanks to the iCOquit®, patients can quickly and easily monitor their CO levels at home to receive instant results on their smartphone or tablet, and share them directly with their Stop Smoking Advisor. This means they can receive instant CO validation of smoking status, and Advisors can better maintain provision of stop smoking medication in addition to providing remote behavioural support.
Jason Smith, Managing Director at Bedfont Scientific Ltd, explains, “The Smokerlyzer range of CO monitors has been helping people quit smoking in clinics for over 40 years. With the evolving healthcare markets and improvements in personal healthcare technology, we wanted to put all those years of experience into creating a device for people to use anytime, anywhere, so they could really invest in their quit smoking attempts in-between their Stop Smoking consultations. We are now working alongside several Key Opinion Leaders to put together educational resources to help people adapt to remote CO monitoring, and with the iCOquit®, it couldn’t be easier.”
To see how the iCOquit®️ is helping with remote CO monitoring, watch our video here: https://youtu.be/f3bSLspUVP8

-ends-
References
- What are the health risks of smoking? [Internet]. nhs.uk. 2021 [cited 1 February 2021]. Available from: https://www.nhs.uk/common-health-questions/lifestyle/what-are-the-health-risks-of-smoking/#:~:text=Smoking%20is%20one%20of%20the,than%2050%20serious%20health%20conditions.
- Shahab L, West R, McNeill A. A randomized, controlled trial of adding expired carbon monoxide feedback to brief stop smoking advice: Evaluation of cognitive and behavioral effects. Health Psychology. 2011;30(1):49-57.