A history of Fractional exhaled Nitric Oxide (FeNO) testing: where it all began
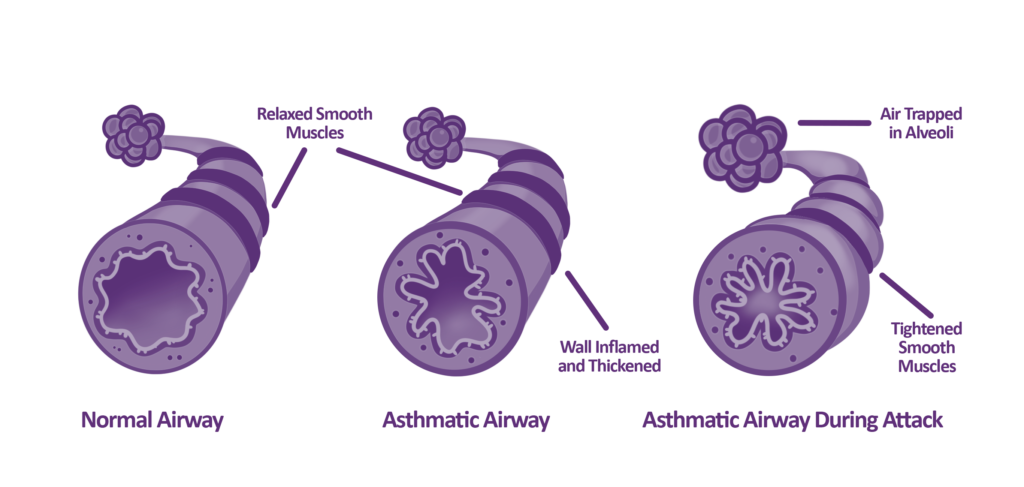
The story of FeNO began in the 1990s after it gained a lot of interest from researchers in the potential it posed as a non-invasive biomarker for airway inflammation. When airways are inflamed, Nitric Oxide (NO) is naturally produced by your body to help combat inflammation. This production of NO was observed by researchers to be significantly higher in patients with asthma. Researchers initially used a technology called ‘chemiluminescence’, to undertake research into FeNO and asthma. Over time, as FeNO testing evolved, so did available technologies on the market, and FeNO testing with electrochemical sensors was introduced as a more robust and cost-effective solution.
Chemiluminescence vs electrochemical FeNO technology: why electrochemical sensor technology is now considered ‘gold standard’
Both chemiluminescence and electrochemical sensor technology is adopted as a means of measuring and quantifying levels of nitric oxide in exhaled breath. Although both technologies are incredibly accurate and reliable when measuring exhaled nitric oxide, they both have note-worthy differences9. Whilst considered a highly sensitive and specific method for testing exhaled nitric oxide, chemiluminescence technology has some significant drawbacks, including the costly nature of the technology, and the additional complexity of using chemiluminescence devices9.
Chemiluminescence technology often requires additional specialist training as well as extra requirements for regular maintenance and calibration, which can lead to significant hikes in operational costs. Additionally, the size and portability of chemiluminescence devices are often at a disadvantage, as devices tend to be very bulky and less portable in comparison to other FeNO technologies such as electrochemical FeNO technology9.
Because of the difficulties chemiluminescence technology presented for widespread adoption in clinical practice, electrochemical technology was considered an alternative technology for carrying out FeNO testing in secondary and primary care. A number of studies were carried out comparing chemiluminescence technology to electrochemical technology, which found that there was a good correlation between the two technologies. A revolutionary finding, due to the cost-effective, accurate and portable nature of electrochemical technology for FeNO testing.
Electrochemical Technology and NObreath®: Dawn of A New Era
The need for more cost-effective, portable, and accurate solutions for FeNO testing was found in electrochemical technology, and a flurry of innovation from med-tech industries ensued, the NObreath® was born.
The NObreath® was developed by Bedfont® Scientific Ltd. in 2008, reflecting on over 10 years of FeNO development. Taking into consideration any obstacles current FeNO technology highlighted on market, the aim for Bedfont® was to develop the ultimate FeNO test solution, creating an electrochemical FeNO device developed with health care providers and patients in mind.
NObreath® vs alternative electrochemical FeNO technologies on the market: are they just as accurate?
NObreath® has been developed with accuracy and repeatability in mind and has been subject to the stringent processes of CE, FDA, CFDA and PMDA clearance (to name but a few) as part of their respective product registration and have also been shown in clinical research to result in excellent repeatability, reproducibility and comparability.
Additionally, the NObreath® device’s electrochemical sensor has been validated against chemiluminescence technology and has shown a good correlation between both technologies7. The NObreath® has been subject to many clinical studies and case study write-ups proving its accuracy and repeatability.
Further to clinical studies, case studies, and scrutiny by a number of different regulatory bodies, NObreath® has been subject to a number of lab condition tests to ensure accuracy, repeatability and stability of the electrochemical sensor, for up to 29,000 tests*, giving patients and healthcare professionals continued and accurate use with the NObreath®.
Finally, in addition to FeNO testing being a widely adopted test for airway inflammation in asthma patients such as ATS and ERS FeNO guidelines1, the NObreath® is one of three FeNO devices recommended by NICE5, an independent international organisation responsible for driving improvement and excellence in the health and social care system.
NObreath®: Breaking barriers in innovation and accessibility for all
NObreath® breaks barriers with its innovative features, making NObreath® the device of choice for healthcare providers.
Instant results
Why wait? Save precious clinic time with the NObreath® by receiving an instant and accurate FeNO test result.
‘No nonsense’ pricing for a cost-effective solution
NObreath® prides itself on being the most cost-effective FeNO solution on the market, by providing competitive pricing for mouthpieces and NObreath® devices. This is in addition to a long shelf life for mouthpieces, making test per patient the most cost-effective solution for operational overheads, increasing accessibility to all.
Incentive flow rate
The NObreath® has a selection of incentive flow rates suitable for all ages, to ensure patients exhale to a flow rate of 50ml/s for optimal and accurate FeNO testing.
FeNO testing without limits
The NObreath® has been designed to ensure continued use, meaning your device can be used over and over again**, reducing cost to your clinic, and limiting wastage for better environmental sustainability. Furthermore, to ensure continued use of your NObreath®, our easy ‘plug and play’ components mean healthcare professionals can easily maintain the NObreath® on-site without having to delay or suspend clinics due to off-site servicing or delay in having to purchase a new FeNO device.
Integrated infection control
The NObreath® device has integrated antimicrobial technology, in addition to integrated bacterial and viral filters in the NObreath® mouthpieces for improved infection control. Simple exhalation-only technique.
Simple exhalation-only technique
The exhalation-only technique on the NObreath® makes FeNO testing easy for all. There is no need to inhale through the device, as our partitioning method ensures any ambient NO is removed from the breath sample. As the breath sample enters the NObreath®, the first few seconds are partitioned and vented through the monitor bypassing the sensor chamber. After the partition period has elapsed, the pump will begin to draw the remaining viable sample into the sensor chamber, where the breath sample will be analysed in real-time. As the sensor measures the sample in real-time, by the end of the test, the result is instantly shown onscreen. Removal of potential environmental NO is advised by ‘ATS/ERS recommendations 2005 for standardized procedures for the measurement of exhaled nitric oxide (FeNO) testing’1, so you can have peace of mind that your FeNO result is accurate and dependable. Learn more about our partitioning method here: https://www.nobreathfeno.com/measuring-feno-with-the-nobreath/
Electrochemical technology: The new ‘gold standard’ for FeNO testing
The evidence showing the comparison to the NObreath® electrochemical FeNO device is directly comparable to chemiluminescence technology and other available electrochemical FeNO technology on the market; you can be sure that you own the ultimate FeNO test solution, an easy-to-use exhalation-only device, providing health care professionals with accurate and reliable results, utilising ‘gold standard’ and cost-effective electrochemical technology, with added portability for clinic use, and much more.
Visit https://www.nobreathfeno.com to find out how you can support your patients with FeNO monitoring, with the NObreath® from Bedfont® Scientific Ltd.
*Subject to correct use, maintenance and servicing
** Subject to 29,000 tests
References:
1. American Thoracic Society and European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide. American Journal of Respiratory and Critical Care Medicine. 2005;171(8):912-930.
2. Inoue Y, Sato S, Manabe T, Makita E, Chiyotanda M, Takahashi K, Yamamoto H, Yanagida N, and Ebisawa M. Measurement of exhaled nitric oxide in children: A comparison between NObreath® and NIOX VERO® analyzers. Allergy, asthma and immunology research. 2018;10(5):478-489.
3. Harnan SE, Tappenden P, Essat M, Gomersall T, Minton J, Wong R, Pavord I, Everard M, and Lawson R. Measurement of exhaled nitric oxide concentration in asthma: A Systematic review and economic evaluation of NIOX MINO®, NIOX VERO®, and NObreath®. Health Technology Assessment. 2015;19(82):1-330.
4. Kang SY, Lee SM, and Lee SP. Measurement of fractional exhaled nitric oxide in adults: comparison of two different analysers (NIOX VERO® and NObreath®). Tuberculosis and Respiratory Diseases. 2021;84(3):182-187.
5. National Institute for Health and Care Excellence. Measuring fractional exhaled nitric oxide concentration in asthma: NIOX MINO®, NIOX VERO®, and NObreath®[DG12]. 2014. Available from: https://www.nice.org.uk/guidance/dg12/chapter/5-Outcomes
6. Pisi R, Aiello M, Tzani P, Marangio E, Olivieri D, and Chetta A. Measurement of fractional exhaled nitric oxide by a new portable device: comparison with the standard technique. Journal of Asthma. 2010;47(7):805-809.
7. Antus B, Horvath I, and Barta I. Assessment of exhaled nitric oxide by a new hand-held device. Respiratory Medicine. 2010;104(9):1377-1380.
8. Yune S, Lee JY, Choi DC, and Lee BY. Fractional exhaled nitric oxide: Comparison between portable devices and correlation with sputum eosinophils. Allergy, Asthma and Immunology Research. 2015;7(4);404-408.
9. Maniscalco M;Vitale C;Vatrella A;Molino A;Bianco A;Mazzarella G; M. Fractional exhaled nitric oxide-measuring devices: Technology updateMauro [Internet]. U.S. National Library of Medicine; 2016 [cited 2023 Nov 22]. Available from: https://pubmed.ncbi.nlm.nih.gov/27382340/