Bedfont has seen a high demand for its NObreath® FeNO monitor following Rapid Uptake Products(RUP) programme
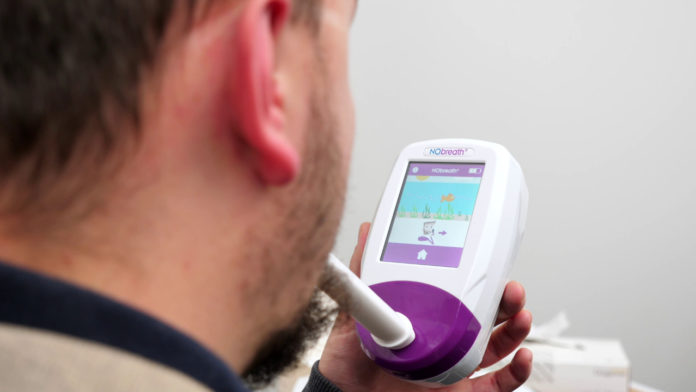
Med-tech company, Bedfont, has seen a rise in demand for its NObreath® FeNO monitor, which aids in the diagnosis and monitoring of asthma, since the airway inflammation measurement tool was included on the Rapid Uptake Products (RUPs) programme.
The RUPs programme designed by the NHS Accelerated Access Collaborative (AAC), identifies products with NICE approval, such asFeNO testing devices, and supports them by helping these innovative products integrate into everyday practice. Thanks to the RUPs programme, NHS organisations were able to apply for funding through the government ‘Pathway Transformation Funding’ (PTF) initiative which helps to cover the costs of devices, consumables and fund other infrastructure requirements to set up FeNO testing (among other services) as part of the patient care pathway.
Jason Smith, Managing Director of Bedfont, said, “Since NICE published guidance relating to FeNO testing some years ago, it has been disheartening to see how many services want to integrate this approved technology into their services but have been unable due to lack of funding and/or education surrounding its use. The AAC have created a platform by which services have been able to secure the vital funding twinned with educational tools.Since applications closed on 30th April 2021, we have seen a high rise in the demand for our FeNO monitors and we are ecstatic to be helping to improve asthma care.”
Bedfont has been fighting to get FeNO monitoring widely accepted worldwide, by raising awareness of measuring FeNO and its benefits for asthma by providing online resources, expert-lead seminars, and a no-nonsense pricing structure.
In collaboration with their UK distributor, Intermedical UK Ltd, Bedfont will provide many devices as part of the programme but are aware that only a small percentage of applications will be successful. For any services that have not been fortunate to secure funding for a NObreath® FeNO device, Intermedical has a limited offer available. Please contact Intermedical on 01732 522444to solve your FeNO monitoring needs today.
