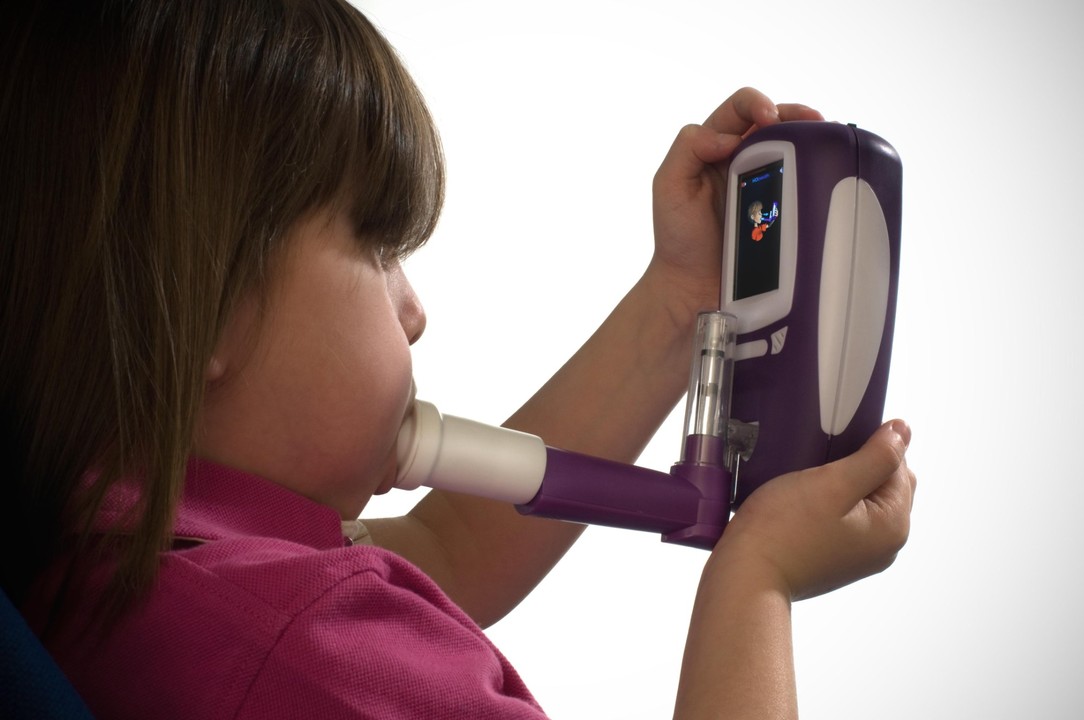
The NObreath® FeNO monitor, recommended by The National Institute for Health and Care Excellence (NICE) [1], is the essential tool for GP’s and asthma patients to help improve asthma management.
According to Asthma UK, currently 5.4 million people suffer from asthma, however, “every day the lives of three families are devastated by the death of a loved one to an asthma attack, and tragically of these deaths are preventable”. In 2016 in England and Wales alone, a shocking 1,237 people died from an asthma attack[2]. In November 2017, NICE published the final guidelines on Asthma: diagnosis, monitoring and chronic asthma management, to improve asthma care, which included the NObreath® FeNO monitor from and second-generation family company, Bedfont Scientific Ltd., as a recommended device.
Bedfont has specialised in the design and manufacture of breath analysis medical devices for over 40 years and in 2017 their NObreath® FeNO monitor is celebrating 10 years of improving asthma management. Bedfont have been campaigning vigorously to raise awareness of FeNO measuring and its benefits, especially after last year when asthma figures sparked increased concern; statistics released from Asthma UK which found that over 120,000 asthma sufferers in the UK are at risk from wrongly prescribed medication and NICE’s findings that 30% of people with asthma suspected to have been misdiagnosed[3,4]. Asthma UK also found that “around 90,000 people in England, 6,000 in Wales, 7,000 in Scotland and 3,500 in Northern Ireland could have uncontrolled asthma that is not being monitored by health-care professionals.”[3]
The NICE asthma guidance has been revised in order to resolve the above concerns, and in particular now includes a patient pathway involving the monitoring of FeNO levels at an early stage to help improve basic asthma care, prevent further misdiagnosis of asthma and the wrong prescription of medication. The new guidelines state that FeNO measurements should be considered ‘as an option to support asthma management in people who are symptomatic despite using inhaled corticosteroids’[5].
Bedfont has responded to the consultation of the NICE asthma guidance and has been working with GP’s on FeNO testing to raise awareness of the benefits now that the NICE asthma guidance recommends FeNO testing in asthma management. Bedfont hopes to help improve basic asthma care and save future lives with the NObreath®, which is also to ATS and ERS guidelines[6]. The NObreath® works by measuring FeNO through breath analysis, making the process quick, simple and non-invasive for both the GP and the patient. Interpreting FeNO levels aids in identifying patients who do/do not require on-going treatment[7] whilst also differentiating between allergic (eosinophilic) and non-allergic asthma[8], and if used daily, FeNO measurements can help to predict exacerbations and attacks.[9]
Jason Smith, Managing Director at Bedfont, explains, “Using FeNO measurements to evaluate airway inflammation in asthma represents a significant advance in respiratory medicine that had been expensive to deliver in everyday practice, until now with the NObreath®. We are honoured to have worked alongside NICE to help improve basic asthma care. The NICE guidelines are often practised and followed around the world and now the guidance on asthma has been published, we expect countries to follow suit and measure FeNO concentration when assessing potential asthma suffers, helping to save countless lives. We will continue innovating and improving health and hope to have a homecare FeNO device in the future, which will enable asthma patients to monitor their FeNO levels anytime, anywhere, to continue improving asthma management one breath at a time.”